Biogeochemical cycles, also known as nutrient cycles, describe the movement of chemical elements through different media, such as the atmosphere, soil, rocks, bodies of water, and organisms. Biogeochemical cycles keep essential elements available to plants and other organisms. The biogeochemical cycles of four elements—carbon, nitrogen, phosphorus, and sulfur—are discussed below. The cycling of these elements is interconnected with the Water Cycle For example, the movement of water is critical for the leaching of sulfur and phosphorus into rivers, lakes, and oceans.
The Carbon Cycle Carbon is the basic building block of all organic materials, and therefore, of living organisms. The carbon cycle is actually comprised of several interconnected cycles: one dealing with rapid carbon exchange among living organisms and the other dealing with the long-term cycling of carbon through geologic processes.
Carbon dioxide in the atmosphere is converted to organic carbon through photosynthesis by terrestrial organisms (like trees) and marine organisms (like algae).
Respiration by terrestrial organisms (like trees and deer) and marine organisms (like algae and fish) release carbon dioxide back into the atmosphere. Additionally, microbes that decompose dead organisms release carbon dioxide through respiration.
Weathering of terrestrial rocks also brings carbon into the soil. Carbon in the soil enters the water through leaching and runoff. It can accumulate into ocean sediments and reenter land through uplifting. Long-term storage of organic carbon occurs when matter from living organisms is buried deep underground and becomes fossilized. Volcanic activity and, more recently, human emissions stored carbon back into the carbon cycle.
The Nitrogen Cycle
All organisms require nitrogen because it is an important component of nucleic acids, proteins, and other organic molecules. Getting nitrogen into living organisms is difficult. Plants and algae are not equipped to incorporate nitrogen from the atmosphere (where it exists as tightly bonded, triple covalent N2) although this molecule comprises approximately 78 percent of the atmosphere. Because most of the nitrogen is stored in the atmosphere, the atmosphere is considered a reservoir of nitrogen.
In the nitrogen cycle, nitrogen-fixing bacteria in the soil or legume root nodules convert nitrogen gas (N2) from the atmosphere to ammonium (NH4+).
Nitrogen-fixing cyanobacteria are essential to maintaining the fertility of semi-aquatic environments like rice paddies. Although the first stable product of the process is ammonia, this is quickly incorporated into protein and other organic nitrogen compounds.
Ammonium is converted by bacteria and archaea into nitrites (NO2−) and then nitrates (NO3−) through the process of nitrification. Like ammonium, nitrites and nitrates are found in water and the soil. Some nitrates are converted back into nitrogen gas, which is released into the atmosphere. The process, called denitrification, is conducted by bacteria. Plants and other producers directly use ammonium and nitrates to make organic molecules through the process of assimilation. This nitrogen is now available to consumers. Organic nitrogen is especially important to the study of ecosystem dynamics because many processes, such as primary production, are limited by the available supply of nitrogen. Consumers excrete organic nitrogen compounds that return to the environment. Additionally dead organisms at each trophic level contain organic nitrogen. Microorganisms, such as bacteria and fungi, decompose these wastes and dead tissues, ultimately producing ammonium through the process of ammonification.
The Phosphorus Cycle Several forms of nitrogen (nitrogen gas, ammnoium, nitrates, etc.) were involved in the nitrogen cycle, but phosphorus remains primarily in the form of the phosphate ion (PO43-). Also in contrast to the nitrogen cycle, there is no form of phosphorus in the atmosphere. Phosphorus is used to make nucleic acids and the phospholipids that comprise biological membranes.
Phosphate enters the atmosphere from volcanic aerosols, which precipitate to Earth. Weathering of rocks also releases phosphate into the soil and water, where it becomes available to terrestrial food webs. Some of the phosphate from terrestrial food webs dissolves in streams and lakes, and the remainder enters the soil. Phosphate enters the ocean via surface runoff, groundwater flow, and river flow, where it becomes dissolved in ocean water or enters marine food webs. Some phosphate falls to the ocean floor where it becomes sediment. If uplifting occurs, this sediment can return to land.
The Sulfur Cycle Sulfur is an essential element for the molecules of living things. As part of the amino acid cysteine, it is critical to the three-dimensional shape of proteins.
Atmospheric sulfur is found in the form of sulfur dioxide (SO2), which enters the atmosphere in three ways:
Sulfur dioxide (SO2) from the atmosphere is dissolved in precipitation as weak sulfuric acid or falls directly to Earth as fallout. This releases sulfates (SO42-) into the soil and water. Soil sulfates can be carried as runoff into the water. Marine sulfate can form pyrite, and this can break down to release soil sulfates.
Organisms in terrestrial and marine ecosystems assimilate sulfate, adding sulfur to organic molecules, such as proteins (not shown). Decomposition of these organisms returns sulfates to the soil. Microorganisms can convert sulfates to hydrogen sulfide (H2S) and vice versa. Decomposition, volcanic eruptions, and human activities (including burning fossil fuels) can release hydrogen sulfide (H2S) or sulfur dioxide into the atmosphere.
Ecological succession
Ecological succession is the gradual and sequential replacement of one community by the other in an area over a period of time. According to E.P. Odum (1971), the ecological succession is an orderly process of community change in a unit area. It is the process of change in species composition in an ecosystem over time.
Process of Ecosystem Succession The ecological succession is a complex process and it may take thousands of years. Frederic Clements in 1916 for the first time proposed the sequential phases of an ecological succession.
Different Types of Ecological Succession
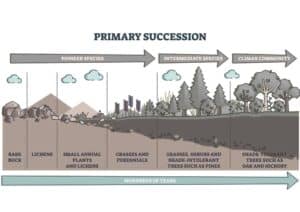
Primary succession is initiated when a new area that has never previously supported an ecological community is colonized by plants and animals. This could be on newly exposed rock surfaces from landslides or lava flows.
Secondary succession occurs when an area that has previously had an ecological community is so disturbed or changed that the original community was destroyed and a new community moves in. This is more common than primary succession and is often the result of natural disasters, such as fires, floods, and winds, as well as human interference, such as logging and clear-cutting.
Stages of Succession
Nudation/Bare Area: The creation of a lifeless area, whether from a disturbance or new habitat formation.
Migration: The dispersal of seeds, spores, and other propagules from existing communities into the bare area by wind, water, or animals.
Ecesis: The successful establishment and growth of the colonizing species as they adapt to the new environment.
Aggregation: The growth in population of these initial species, leading to a denser and more complex community.
Competition and Co-action: As populations grow, species begin to interact and compete for resources, influencing the community’s structure.
Reaction: The development of new environmental conditions by the existing community that may hinder its own survival but facilitate the establishment of new species.
Stabilization/Climax Community: A stable, mature community that is relatively well-adapted to its environment, with high biodiversity and a balanced species composition.